The Role of Neutron Moderators in Nuclear Reactors: Principles, Materials, and Applications
In nuclear engineering, neutron moderators are materials that reduce the speed of fast neutrons, converting them into thermal neutrons capable of initiating the uranium-235 chain reaction. The most common moderators include light water, graphite, and heavy water. Moderators are selected based on their proximity to neutron mass, high scattering cross-section, and low absorption cross-section. These materials play a crucial role in the safety and efficiency of nuclear reactors.
Table of Contents
Neutron Moderators
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, converting them into thermal neutrons capable of entering the uranium-235 chain reaction. Common moderators include light water (approximately 75% of reactors), solid graphite (20% of reactors), and heavy water (5% of reactors). Beryllium is also used in some experimental reactors, and hydrocarbons have been proposed as another option. Neutrons are usually bound to atomic nuclei and do not exist freely in nature for long periods. Unbound neutrons have a half-life of about 15 minutes. Releasing neutrons from nuclei requires energy greater than the binding energy of neutrons to the nucleus (typically around 7-9 MeV for most isotopes). Neutron sources produce free neutrons using various nuclear reactions, including fusion and fission. However, neutron sources emit neutrons with energies around MeV. Since kinetic energy, EE, can be related to temperature by the equation: $$E = \frac{3}{2} k_B T$$ the characteristic temperature of a neutron with several MeV is approximately 10 million degrees Celsius.
Moderation
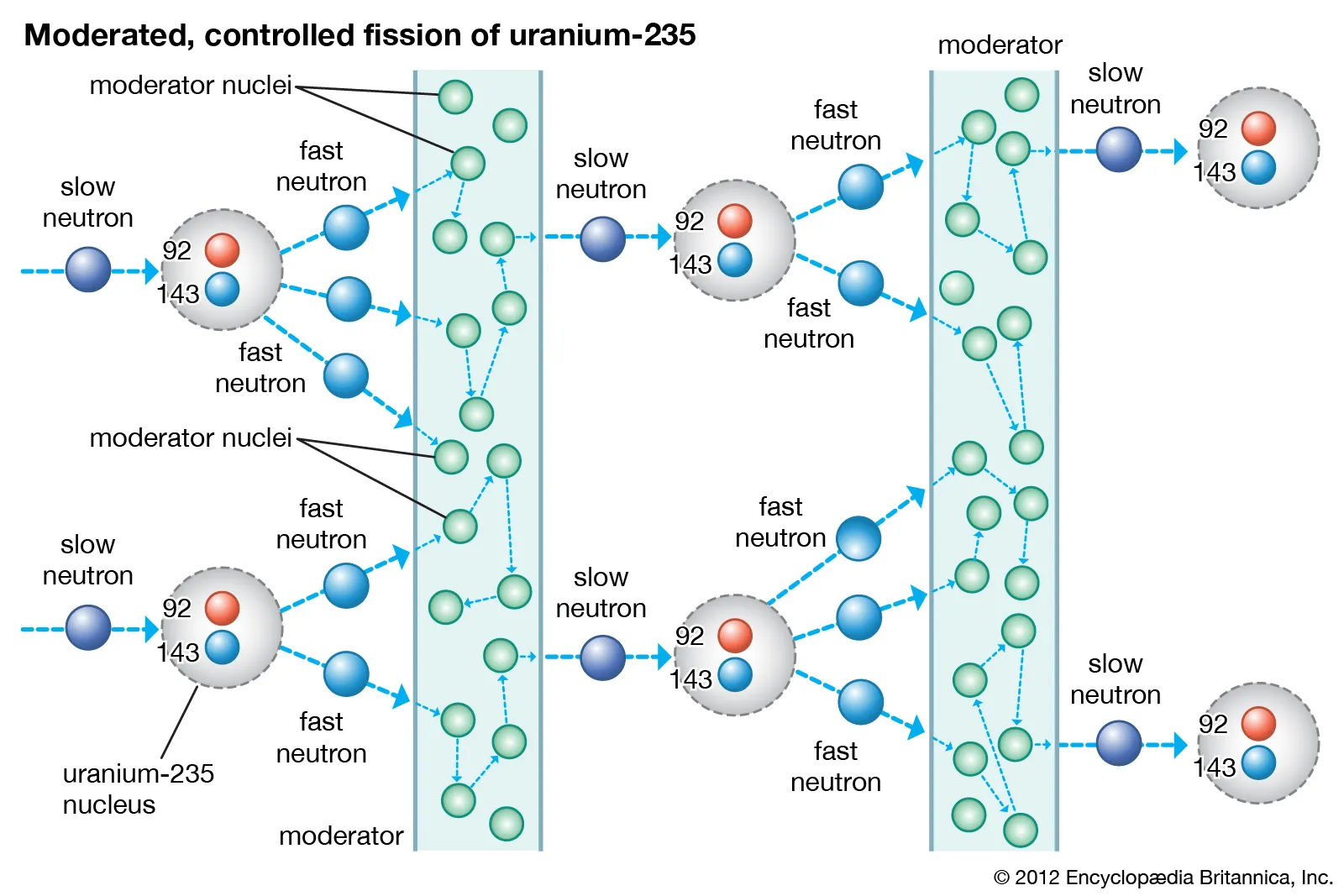
Moderation is the process of reducing the high initial kinetic energy of free neutrons. Since energy is conserved, this reduction of neutron kinetic energy is achieved by transferring energy to a material known as a moderator. This material is also known as a neutron decelerator because it simultaneously reduces energy and speed. The probability of neutron scattering from a nucleus is given by the scattering cross-section. The first collision of a neutron with a moderator may have sufficiently high energy to excite the moderator nucleus. Such a collision is inelastic, as part of the kinetic energy is converted to potential energy by exciting some internal degrees of freedom to form an excited state. As neutron energy decreases, collisions become mostly elastic; the total energy and momentum of the system (neutron and nucleus) remain conserved. From the mathematics of elastic collisions, neutron moderation is most efficient when the moderator nuclei have masses close to that of the neutron.
Selection of Moderator Materials
A neutron colliding with a mass-1 nucleus (such as a proton) can potentially transfer all its energy in a head-on collision. For general cases, both head-on and other collision types must be considered. The logarithmic average energy loss of a neutron per collision, ξ\xi, depends only on the atomic mass AA of the nucleus and is given by: $$\xi = \frac{4A}{(A+1)^2}$$ which can be approximated by: $$\xi \approx \frac{2}{A+1}$$ From this, the expected number of neutron collisions with a given type of nucleus to reduce neutron kinetic energy from E0E_0 to EE can be calculated.
Selection of Moderator Materials
Some nuclei have larger absorption cross-sections than others, removing neutrons from the flux. Thus, an effective moderator should have a small absorption cross-section. The efficiency of moderation is the ratio of the microscopic scattering cross-section σs\sigma_s weighted by ξ\xi to the microscopic absorption cross-section σa\sigma_a, i.e.: $$\frac{\sigma_s \cdot \xi}{\sigma_a}$$
Neutron Speed Distribution
After sufficient collisions, the neutron speed will be comparable to the speed of nuclei given by thermal motion; this neutron is called a thermal neutron, and the process is referred to as thermalization. At equilibrium at a given temperature, the expected speed (energy) distribution of elastic scattering of hard spheres is given by the Maxwell-Boltzmann distribution. Due to the speed (energy) dependent absorption cross-section of most materials, a slight correction is needed in real moderators, with slower neutrons being absorbed earlier, resulting in a neutron speed distribution in the core slightly sharper than predicted.
Reactor Moderators
In thermal nuclear reactors, the core of a heavy fuel element such as uranium absorbs a slow-speed free neutron, becoming unstable and splitting into two smaller atoms (fission fragments). The fission process for uranium-235 produces two fission fragments, two or three fast free neutrons, and initial energy manifesting as the kinetic energy of the recoiling fission fragments. Fast free neutrons are emitted with kinetic energy around 2 MeV. Since most neutrons from a uranium fission event are emitted as fast neutrons, which require thermal neutrons to initiate fission, the reaction can be self-sustaining under controlled conditions, releasing significant energy. The likelihood of further fissions is determined by the fission cross-section, which depends on the speed (energy) of the incident neutrons. In thermal reactors, high-speed neutrons around MeV are less likely to cause fission. (While it is not impossible for fast neutrons to cause fission, it is less probable). New fast neutrons emitted, moving at about 10% the speed of light, must be slowed or "moderated" from speeds of several kilometers per second if they are to have a higher likelihood of causing fission near uranium-235 nuclei, ensuring the continuation of the chain reaction.
Moderator Contaminants
Good moderators should have low concentrations of neutron-absorbing impurities like boron. In commercial nuclear power plants, moderators typically contain dissolved boron. The concentration of boron in the reactor coolant is manually adjusted by operators by adding boric acid or diluting with water to control plant power. During World War II, the German nuclear program encountered a major obstacle when cheap graphite moderators failed. At the time, most graphite was deposited on boron electrodes, and German commercial graphite contained high levels of boron. Since the wartime German program could not solve this issue, they were forced to use expensive heavy water moderators. In the United States, physicist and chemical engineer Leó Szilárd solved this problem.
Non-Graphite Moderators
Some moderators, like beryllium and heavy water, are very expensive. Heavy water must be 99.75% pure to react with natural (unenriched) uranium. This is challenging because heavy water and regular water have similar chemical bonds, differing only slightly in their rates. Cheaper light water (very pure regular water) absorbs too many neutrons to be used with unenriched uranium, requiring enriched uranium or nuclear fuel reprocessing for the operation of such reactors. Both enrichment and reprocessing are technically complex and expensive processes, and several methods of enrichment and reprocessing can be used to produce material usable in nuclear weapons.
CANDU Reactors
CANDU reactors double safety with a low-temperature, low-pressure heavy water tank that moderates neutrons and also acts as a heat sink in the event of coolant loss. This system is separate from the fuel rods generating heat. Heavy water is very effective at neutron moderation, thus enabling the characteristic "neutron economy" of CANDU reactors.